Case Study Overview
Objective
To investigate the factors affecting the rate of chemical reactions using the dissolution of sodium bicarbonate as a model, and to apply these findings to real-world industrial challenges, such as ammonia synthesis.
Challenge
Maintaining experimental rigor while using non-laboratory equipment to assess dissolution kinetics under three variables (temperature, mass, granularity). Critical hurdles included precise thermal control without calibrated baths (±5°C variance), manual mass measurement errors (±0.3g tolerance), and compensating for solute substitution (vitamin C vs pure NaHCO3). Success depended on standardizing visual dissolution endpoints across trials while accounting for foam interference and colourant dissolution rates in household glassware.
Proud Achievement: National Challenge Winner!
I am thrilled to share that this assignment earned first place in the National Challenge! This recognition reflects the hard work, dedication, and innovative thinking that went into exploring the fascinating world of chemistry and its real-world applications. Thank you to everyone who supported this journey!

Context
Chemistry in Everyday Life
We know that Chemistry is a subject studied from the early years of school to advanced topics in undergraduate degrees. But have you ever stopped to think about how important chemistry is in our lives?
“Chemical Science is not just about discovery. It is also, and especially, about creation and transformation. Without the work of chemists throughout history, some spectacular achievements would never have happened, such as advances in the treatment of diseases, space exploration, and the wonders of modern technology.
Chemistry makes an essential contribution to humanity with food and medicines, clothing and shelter, energy and raw materials, transport, and communications. It also provides materials for Physics and industry, models and substrates for Biology and Pharmacology, and properties and procedures for other sciences and technologies.
Thanks to Chemistry, our world has become a more comfortable place to live. Our cars, homes, and clothes overflow with chemical creativity. Our energy future will depend on Chemistry, as will achieving one of the Millennium Goals, which is to provide safe water and basic sanitation for all humanity.
A world without Chemical Science would be a world without synthetic materials, meaning no phones, computers, or cinema. It would also be a world without aspirin or detergents, shampoo or toothpaste, without cosmetics, contraceptives, or paper—and thus no newspapers or books, glue, or paints. In short, without the development provided by Chemical Science, life today would be dull, short, and painful!”
ZUCCO, C. Chemistry for a Better World. Scielo Brazil, 2011.
Ammonia Synthesis
Ammonia (NH3) is one of the most produced organic chemicals, composed of nitrogen and hydrogen gases. The reaction for the formation of ammonia involves high temperature and pressure in the presence of a catalyst. It is the simplest stable compound of these elements and serves as a starting material for the production of many commercially important nitrogen compounds. Most of the ammonia produced is used as fertiliser, although it can also be used in the manufacture of explosives.
Experiment: Rate of Chemical Reactions – Dissolution of Sodium Bicarbonate
Materials:
- 6 glass containers of the same size (e.g., a glass).
- 6 effervescent tablets (antacid, “fruit salt,” sodium bicarbonate).
- Water.
Experimental Procedure:
Test 1:
- Add room-temperature water to one container and hot water (not boiling) to another.
- At the same time, add one effervescent tablet to each container.
- Observe and record the conditions.
Test 2:
- Add room-temperature water to two containers.
- At the same time, add one whole effervescent tablet to one container and half a tablet to the other.
- Observe and record the conditions.
Test 3:
- Crush one effervescent tablet.
- Add the same amount of water to two containers.
- At the same time, add one whole effervescent tablet to one container and the crushed tablet to the other.
- Observe and record the conditions.
Note: Ammonia synthesis is more complex and involves numerous variables. For this reason, it has been simplified and compared to this experiment for educational purposes only.
Rate of Chemical Reactions – Dissolution of Sodium Bicarbonate
1. Introduction
According to Souza, sodium bicarbonate is a crystalline mixture, soluble in water, with an alkaline taste. When heated, it decomposes, releasing carbon dioxide, which explains its use as a chemical leavening agent in food preparation. Its most well-known use is as a stomach antacid, as the product of its reaction with water is sodium hydroxide (NaOH), which neutralises stomach acid (HCl). Additionally, it has various applications in different fields, such as: laboratory reagent, gold and platinum electroplating, tanning, wool and silk treatment, animal nutrition, ceramics, butter and wood preservation, cosmetics, and fire extinguisher production.
The aim of this experiment is to analyse the dissolution rate of sodium bicarbonate in the form of effervescent tablets under different conditions. Three experiments were conducted: in the first, the solvent temperature was varied; in the second, different solute masses were used; and in the third, the solute's granularity was modified. The experiments were conducted at room temperature (approximately 25°C) and at sea level (1 atm pressure), i.e., under standard temperature and pressure conditions (STP).
According to Polachini (2015), an effervescent tablet consists of a compressed medication, a mixture of weak organic acids, and carbonate bases, primarily sodium bicarbonate. When in contact with an aqueous solvent, such as water, the acid molecules ionise, forming carbonic acid (H2CO3), which decomposes due to its instability, releasing carbon dioxide (CO2) bubbles into the atmosphere.
The equipment used was not precision-grade but rather common household items. Temperature and mass measurements were approximate, as the experiment was conducted for educational purposes. However, a study with precision laboratory equipment would be necessary to confirm the results. High-precision stopwatches were used to measure reaction rates.
2. Experimental Procedure
2.1 Materials and Methods
- 6 glass beakers
- Measuring cylinder
- Kitchen scale
- Pestle and mortar
- Digital thermometer
- Stopwatches
- Greaseproof paper
- Knife
- Spoon
- Pan
- Electric hot plate
- 600 ml of distilled water
- 6 effervescent sodium bicarbonate tablets (vitamin C)
Picture 1: Utilised materials

Experiment 1 – Different Temperatures
- Measure 100 ml of distilled water using a measuring cylinder.
- Transfer the water to a pan and heat it on an electric hot plate for 5 minutes.
- Transfer the heated water to a glass beaker and measure its temperature using a digital thermometer (70.4°C).
- Measure another 100 ml of distilled water and transfer it to a second beaker (room temperature: 25.6°C).
- Prepare two stopwatches.
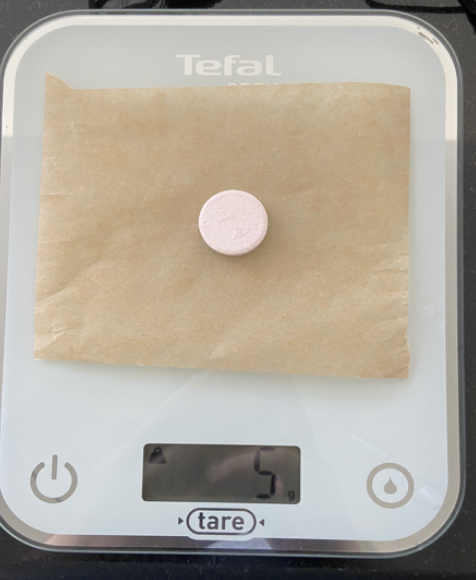
- Weigh two effervescent vitamin C tablets (5 g each).
- Simultaneously place one tablet in each beaker and start the stopwatches.
- Observe the reaction and stop the stopwatches once the tablets have fully dissolved.
Picture 2: Measuring distilled water

Picture 3: Temperature calibration

Picture 4: Solute weighing
Experiment 2 – Different Solute Masses
- Measure 100 ml of distilled water and transfer it to two glass beakers.
- Cut one effervescent tablet in half, leaving the other whole.
- Weigh both tablets (5 g for the whole tablet and approximately 2.5 g for the half tablet).
- Prepare the stopwatches.
- Simultaneously place the tablets in the beakers and start the stopwatches.
- Observe the reaction and stop the stopwatches once the tablets have fully dissolved.
Picture 5: Solute weighing

Picture 6: Measuring distilled water
Experiment 3 – Different Solute Granularities
- Measure 100 ml of distilled water and transfer it to two glass beakers.
- Crush one effervescent tablet using a pestle and mortar, leaving the other whole.
- Weigh both tablets (5 g each).
- Prepare the stopwatches.
- Simultaneously place the tablets in the beakers and start the stopwatches.
- Observe the reaction and stop the stopwatches once the tablets have fully dissolved.
Picture 7: Triturating the solute

Picture 8: Measuring distilled water

Picture 9: Solute weighing

3. Results and Discussion
The solute was replaced with vitamin C due to the unavailability of effervescent sodium bicarbonate tablets in the locality where the experiment was conducted. This substitution was recommended by the supervising teachers.
According to the manufacturer, the solute's composition includes ascorbic acid, sodium bicarbonate, sorbitol, tricalcium phosphate, aspartame, acesulfame, and colouring agents. For simplicity, only sodium bicarbonate (NaHCO3) was considered in the reaction.
The reaction between sodium bicarbonate and water produces sodium hydroxide and carbonic acid, which decomposes into carbon dioxide (CO2):
NaHCO3 (s) + H2O (l) → NaOH (aq) + H2CO3 (g)
In all experiments, 100 ml of distilled water was used as the solvent.
Experiment 1: The temperature variation significantly influenced the dissolution rate. At 25.6°C, 5 g of solute took 1 minute 13.78 seconds to dissolve, while at 70.4°C, it took only 55.88 seconds. The higher-temperature solution produced less foam and appeared more transparent.
Graph 1: Experimental data showing the correlation between solvent temperature and dissolution rate/h2>
Picture 10: Experiment 1 in progress

Picture 11: Variation in solution color

Experiment 2: Reducing the solute mass by 50% (to 2.5 g) resulted in a 31% reduction in dissolution time. The solution's colour was also lighter due to the reduced amount of colouring agent.
Picture 12: Experiment 2 in progress

Graph 2: Experimental data showing the correlation between solute concentration and dissolution rate
Experiment 3: Crushing the tablet increased the dissolution rate by 42%, as the smaller particles reacted more quickly with the solvent. This also produced more foam compared to the other experiments.
Picture 13: Experiment 3 in progress

Graph 3: Experimental data showing the correlation between solute particle size and dissolution rate
4. Conclusion
It was concluded that increasing the solvent temperature accelerates the dissolution of effervescent sodium bicarbonate tablets, as higher temperatures increase particle agitation and reaction rates. Reducing the solute mass by 50% decreased the reaction time by 31%, while crushing the tablet increased the dissolution rate by 42% due to the larger surface area.
Graph 4: Comparison of dissolution rates in all experiments
Relating these findings to ammonia synthesis, the issue may lie in the temperature and pressure conditions required for the reaction. Increasing these parameters would likely speed up the synthesis. Another possible cause is the inadequate concentration of reactants. According to Bigatão (2019), ammonia synthesis is a reversible reaction, meaning the reaction reaches equilibrium, and the product can revert to reactants. The reaction rate is proportional to the concentration of reactants and products.
The balanced equation for ammonia synthesis is:
N2 (g) + 3H2 (g) ⇌ 2NH3 (g)
Picture 14: llustrative graph of the equilibrium point in ammonia synthesis
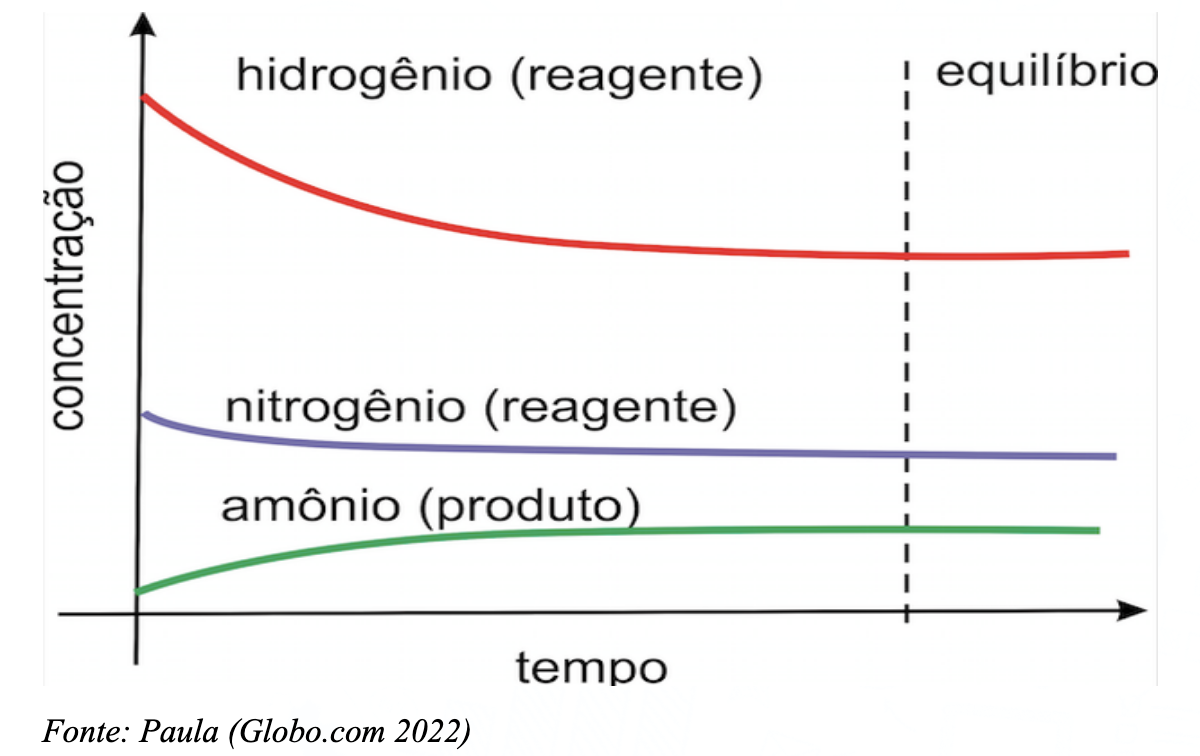
Other factors, such as the physical state of reactants and the presence of catalysts, may also influence the reaction rate. However, temperature, pressure, and reactant concentration are the most likely culprits in this case.
5.0 References
BIGATÃO, Denise Maria Malachini Miotto. Química Geral e Inorgânica. Maringá-PR.: Unicesumar, 2019.
GODOI, Thiago. Química Experimental. Maringá-PR.: Unicesumar, 2019.
PAULA, Camila Salgado de. Equilíbrio Químico e Constante de Equilíbrio. [S.l.]: Globo.com, 2022. Disponível em: http://educacao.globo.com/quimica/assunto/equilibrio-quimico/equilibrio-quimico-e-constante-de-equilibrio.html. Acesso em: 10 set. 2022.
POLACHINI, Marcelo. Como funciona um remédio efervescente? Revista Superinteressante, 2015. Disponível em: https://super.abril.com.br/mundo-estranho/como-funciona-um-remedio-efervescente/. Acesso em: 04 de setembro de 2022.
SOUZA, Líria Alves de. Bicarbonato de Sódio. Brasil Escola. Disponível em: https://brasilescola.uol.com.br/quimica/bicarbonato-de-sodio.htm. Acesso em: 04 de setembro de 2022.
Annex

NATIONAL CHALLENGE NOTICE
The Distance Education Centre (NEaD) of UniCesumar hereby announces, for the attention of interested parties, the present notice for the offering of the second edition of the NATIONAL CHALLENGE, to be held in module 53/2022.
1. Purpose
The purpose of this notice is the execution of the National Challenge through Professional Environments, providing meaningful learning, with the primary focus being the immersion of students in the professional field through the MAPA activity. In this activity, students will be required to present innovative solutions and decision-making for a real or fictional problem.
A professional environment is understood as the actual workplace that enables students to experience practical and meaningful learning through direct interaction with more experienced professionals in the full exercise of specific work activities. To understand and learn more about this opportunity, visit the official National Challenge website1 and follow the key information.
2. Participation
- Students enrolled in module 53/2022 at UniCesumar may participate in the National Challenge,
provided their discipline is included in the project (National Challenge).
https://sites.google.com/unicesumar.com.br/desafionacional-532022/página-inicial - Students enrolled in fully online distance learning (EAD) courses who wish to participate must enrol in the 1st discipline (or concurrently) of module 53/2022.
- Students enrolled in hybrid courses will have a pre-selected discipline; however, their participation, submission, and completion of the activity will only be enabled during the course of their studies.
- To participate, the student must complete the MAPA activity using the National Challenge template form within the deadline, adhering to the instructions and criteria established in the activity.
- In addition to the MAPA activity, it is mandatory for the student to complete all activities of the discipline participating in the National Challenge.
- The student’s participation must be immersive in the professional environment corresponding to their course.
- Students choosing to complete the activity in a professional environment must sign a Partnership Agreement and a Commitment Agreement, which require validation by the responsible department at UniCesumar.
- Participation in the National Challenge will be free of charge for students.
- Students, regardless of their course and year of study, may participate in the challenge, but they cannot compete with disciplines enrolled under a dependency regime, only curricular and/or adaptation.
- Students who are employees of the institution (IES) may not participate in the Challenge.
3. Registration
- Registration implies full awareness and acceptance of the rules and conditions set out in this notice, as well as other rules pertaining to the period designated for the National Challenge.
- To register, the student must access the National Challenge environment. The access link will be available in the discipline environment and can also be accessed via the link: https://forms.gle/MnzLTZCGWaWuBwBH7
- The registration period will be from 18/07/2022 to 29/07/2022.
- The MAPA activity will be released in the discipline environment.
4. Classification
- The top placements from each discipline of UniCesumar’s EAD undergraduate courses will be
classified, observing the following rule:
- Stage 01: Ten students from each discipline included in the national challenge for each course, who achieve the highest score in the Challenge and have completed all evaluative activities of the discipline, will be classified. In the event of a tie, the criteria established in this notice will be observed.
- Stage 02: The ten classified students from each discipline per course will have their MAPAs analysed by UniCesumar’s education professionals (in an odd number), who will select the first-place winner based on the MAPA proposal and student participation.
5. Results
- The results will be made available in the National Challenge environment, with the 1st stage results on 07/11/2022 (Monday) and the 2nd stage results on 21/11/2022 (Monday).
- In the event of a tie, the following criteria will be observed:
- Having registered via the registration form provided on the Challenge website.
- Being active at the institution (IES) at the time of the result announcement.
- Grade received in the discipline’s study activities.
- Having attended the 3 lectures of the General Knowledge Week and responded to the questionnaire.
- Attendance at conceptual classes of the discipline.
- Participation in the forum.
- Student engagement in the course.
- Evaluation by the course board.
6. Awards
- All students participating in the National Challenge will receive a 10-hour complementary certificate after completing their registration via the form available in the Challenge environment and submitting the MAPA activity in the specific template.
- Students who achieve 1st place in each course will be awarded:
- A testimonial from the Course Coordinator on the student’s LinkedIn profile in the recommendations section;
- One voucher for a free course in the EAD Universe;
- Blockchain certification.
7. General Considerations
- Instructions for completing the MAPA for each discipline will be provided in the activity itself, and students may clarify doubts via “contact the mediator”.
- The disciplines Sociocultural and Ethical Formation I and II, GO: Life Project, Opportunity Identification, Opportunity Preparation, Opportunities and Results, the Culinary Skills discipline, Elementary Mathematics, and METEP (online) are not eligible to participate in the Challenge, as they are offered in an online format.
- Students who commit plagiarism, fail to follow the activity instructions, or complete the MAPA without adhering to pedagogical criteria will be disqualified.
- Students who submit the activity after the deadline, with an incorrect, blank, or corrupted file, will be automatically disqualified.
- The MAPA activity provided will be the same for all students, respecting the course and year, and the correction will also be equal, regardless of participation in the National Challenge. The differentiating factor will be the performance of the registered student.
- Situations not defined in this notice will be analysed by the course board and submitted for approval by the Pro-rector of EAD Education.